Cyclohexane Reaction With Bromine
NFPA 704 Marking System The National Fire Protection Association NFPA in section 704 of the National Fire Code specifies a system for identifying the hazards associated with materials. Although the system was developed primarily with the needs of fire protection agencies in.
Properties Of Hydrocarbons Mendelset
Substrate structure controls substitution mechanism S N 1 or S N 2.

. C 6 H 12 Z-hex-3-ene. The Hofmann rearrangement Hofmann degradation is the organic reaction of a primary amide to a primary amine with one fewer carbon atom. A demonstration of bromine substitution and addition reactions is helpful at this point.
This special stability is due to a unique conformation it adopts. C 4 H 4 N 2. 43 Conformation Analysis of Cyclohexane Chair conformation of cyclohexane.
Flinn Scientific is the 1 source for science supplies and equipment both in and outside the classroom. As we will learn many antioxidantscompounds that prevent unwanted radical oxidation reactions from occurringare phenols compounds that contain an OH group bonded directly to a benzene ringa Explain why homolysis of the OH bond in phenol requiresconsiderably less energy than homolysis of the OH bond in ethanol362 kJmol vs. Simple S N 2 reaction.
S N 2 Reaction. Cyclohexane is the most stable cycloalkane. S N 1 and S N 2.
The product is cyclohexane and the heat of reaction provides evidence of benzenes thermodynamic stability. The reaction can form a wide range of products including alkyl and aryl amines. Allyl Chloride with HS S N 2 Reaction.
Figure 81b E2 Reaction Mechanism. Consisting all single bonds. BrF-bromine fluoride anion.
At the same time the β C-H sigma bond begins to move in to become the π bond of a double bond and. Bromine monoxide anion BrO Bromine monoxide cation C v. 2 o Benzyl Chloride with HS S N 2 Mechanism.
The base OH uses its electron pair to attack a β-hydrogen on β-carbon and starts to form a bond. Information contained on this and linked pages comes directly from the 1990 edition of NFPA 704. This is easy to mistake when hurrying so be careful when you are intepreting any structural formulas which include hexagons.
The reaction rates were measured by using cyclohexane k H and cyclohexane-d 12 k D as substrates fig. Stability and structure of carbocations. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate.
A KIE value of 112 k H k D was determined from parallel reactions 25 which indicated that the CHCD activation by Cl was not involved in the turnover-limiting step. Benzyl Chloride with HS S N 2 Reaction. Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atomsThe term unsaturated means more hydrogen atoms may be added to the hydrocarbon to make it saturated ie.
The E2 mechanism is also a single-step concerted reaction similar to S N 2 with multiple electron pair transfers that happen at the same time. Benzene does not react with bromine unless a very bright light or a strong catalyst is used and then the reaction is not an addition reaction. Solution for Consider the reaction of bromine with the pictured alkene.
Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5. C 6 H 12 1R2R-12-dimethylcyclobutane. The configuration of an unsaturated carbons include straight chain such as alkenes and alkynes as well as branched.
It is strain-free meaning neither angle strains nor torsional strains apply and it shows the same stability as chain alkanes. For more than 40 years Flinn has been the Safer Source for Science. It is simply cyclohexane and there are two hydrogens on each carbon atom.
Substituted benzene rings may also be deduced in this fashion and hydroxy-substituted compounds such as phenol catechol and resorcinol give. S N 2 examples.

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange
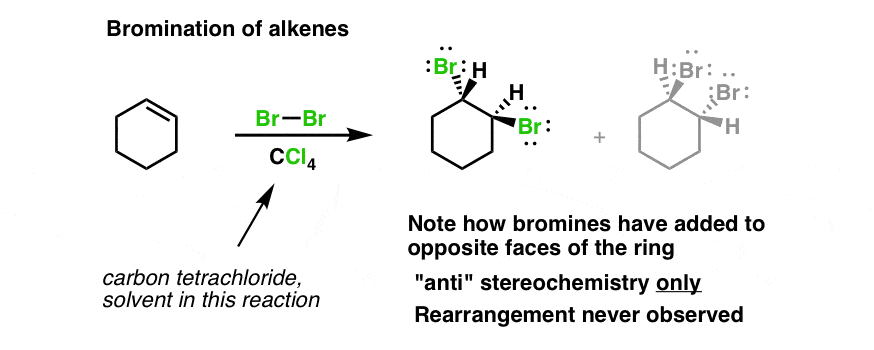
Bromination Of Alkenes Master Organic Chemistry
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene
What Happens When Hexane Reacts With Bromine Quora
0 Response to "Cyclohexane Reaction With Bromine"
Post a Comment